What Is Data?
The specific bits of information that come from research studies are called data. The data that are collected from clinical trials are analyzed and the conclusions are referred to as Clinical Trial Results. The data collected from real-world studies are called real-world data (RWD), which then inform or make up real-world evidence (RWE).
More About Clinical Trials & Real-World Studies
Discussing studies about a medicine with your doctor can help you think through your treatment options. Watch this video and keep reading to learn more.
When you’re looking at treatment options for metastatic breast cancer, it’s important to learn everything you can about the medicine you’re considering.
Understanding studies on how well a medicine works for patients can help you make informed decisions with your doctor.
There are different kinds of research studies. Here, you'll learn about 2 types of studies: clinical trials and real-world studies. You’ll also find out how real-world studies can complement clinical trials.
Let’s take a closer look, starting with clinical trials.
Clinical trials study a medicine’s safety—like its side effects—and efficacy—meaning how well it works.
Clinical trials are considered the gold standard for studying a new or existing medicine.
In fact, the Food and Drug Administration looks at clinical trial results when deciding whether to approve a new medicine in the U.S.
One type of clinical trial is a randomized controlled trial, which compares results for different groups of participants.
Patients participating in a randomized controlled trial must qualify based on strict rules and conditions.
Once accepted into the study, the patients are randomized—meaning assigned at random—to different groups.
In this example, one group of patients receives the medicine being tested, and the control group of patients is not given the medicine being tested.
Information, called data, is collected from these groups and compared to determine results.
Once the trial ends, those results are made public—so you can see how that medicine performed and discuss it with your doctor.
Now onto another type of study: real-world studies.
A real-world study observes patients’ experiences with an approved medicine used in the ‘real world.’ It can be another source of information to look at when deciding on a treatment with your doctor.
A real-world study may include data from patients who have different characteristics—which may not have been included in clinical trials.
This real-world data is captured from sources in everyday medical care. No patient names or identifying information are included in the data, so it is anonymous.
Evidence from these studies, called real-world evidence, can be another source of information about a medicine to discuss with your doctor.
It’s important to note that clinical trials remain the gold standard in studying a medicine’s safety and efficacy. Real-world evidence cannot be directly compared to clinical trials.
So, why is it important to learn about different ways medicines are studied?
- Understanding studies and their data can help give you a greater understanding about a medicine you’re considering.
- Clinical trials are the gold standard for studying a medicine.
- And real-world evidence can give you information about a medicine’s use in the real world.
For answers to questions about data and how medicines are studied, talk to your doctor. Discussions with your doctor can help you make treatment decisions together.
What Clinical Trial Results Can Tell Us
Clinical trials can help us understand a medicine's potential benefits ("efficacy" or how well a medicine works for a specific disease) and risks ("safety" or side effects). They are often run before a new treatment or medicine is reviewed by the Food & Drug Administration (FDA) and are among the important information that the FDA considers when deciding whether to approve a therapy for use in people in the U.S. They are also typically reviewed when the FDA is considering a new use for an already-approved treatment or medicine.

One type of clinical trial is a randomized controlled trial (RCT).
- In this kind of research study, patients are ‘randomized,’ meaning that they are assigned at random (like flipping a coin) to different groups within the study (a randomized study can have 2 or more groups). This way the groups can be better ‘balanced’ so that who is in which group should not influence the results.
- ‘Controlled’ means that the study compares results for the group, or groups, of patients who are given the medicine being tested with results for the group (or groups) that is given something else or no treatment at all (called a control group).

At the end of the clinical trial (and sometimes during), the groups are compared based on the goals that were set before the study started to understand whether the medicine did what it was expected to do and whether there are any safety issues.
Clinical trials give us important information on the efficacy and safety of the medicine, but there are limitations to this type of study. Clinical trials take place under very controlled conditions and have strict rules on who can be included in the study. While these strict rules help us understand if the results that were seen were due to the medicine itself, they may not always represent ‘real-world’ situations or the larger population of people living with the disease being studied.
RCTs are considered the gold standard in studying medications
- RCTs are most often run before a treatment is approved by the FDA, so it can be prescribed to patients.
- Before a trial starts, researchers decide what data needs to be collected to answer specific questions based on the goals of the study. Researchers then collect the data throughout the length of the study so that it can be available for analysis when the study is completed (or sometimes during the study).
- When a trial is controlled, results for the specific group of people taking a medicine are compared to results for another group not taking that medicine.
What Real-World Evidence (RWE) Can Tell Us
Once a medicine is approved by the FDA and more people are using it, more information becomes available. RWE can provide more information on the experience patients have with the medicine in the ‘real world.’ Sometimes this additional information includes data relating to patients that are approved to use the medicine, but have characteristics that are more like those taking the medicine in the ‘real world.’


RWE can complement the RCT information available on approved medications
- Information is gathered after a treatment is approved by the FDA and is being prescribed to patients in the U.S.
- The data that make up RWE are captured from everyday medical care. It can come from various sources, including electronic health records, pharmacy and insurance claims, and patient apps to better understand how patients are using and responding to a medicine once it has been approved for use. No patient names or identifying information are included in this data, so specific patients cannot be identified by the data collected.
- Real-world studies may choose to observe data from people who are similar, but the patient population in RWE can include other characteristics, which may not have been included in a clinical trial.
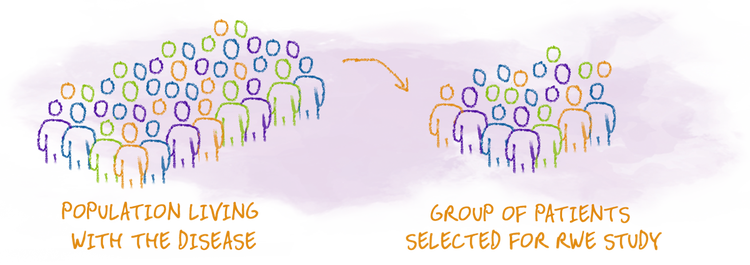
As a reminder, clinical trials remain the gold standard in the development of new medicines, or new uses for already-approved medicines. RWE cannot be directly compared with clinical trials. RWE offers information about a medicine in everyday use and may represent a broader population than those in the clinical trials. However, this data has limitations that are important to know:
- Real-world data are collected from various sources and may be incomplete because researchers can’t go back to collect missing information.
- In the real world, patients are not randomized, so there may be bias in the data collected.
- When considering RWE, remember that it's only based on the patients represented in the data source.
- Safety data may not be collected in real-world studies.
Start A Conversation With Your Doctor
Researching the data about treatment options may leave you with questions—and it’s important to get the answers you need. Talk to your doctor. Being informed can help you actively participate in decisions about your health.
Doctor Discussion Guide
Get the most out of your next visit with your healthcare team. Print out this list of questions to help start the conversation.